Ionic Compound Formula for Barium Phosphate
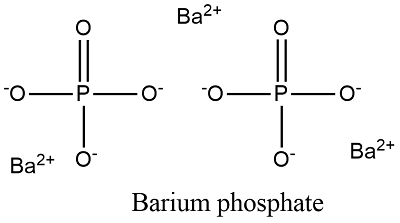
2 Barium Phosphate Potassium SO 4-2 K 2 SO 4 Potassium Sulfate Calcium NO 3-1 CaNO 3 2. The formula for barium phosphate is Ba3 PO42.
The chemical formula for barium phosphate is Ba 3 PO 4 2.

. What is the ionic formula for barium and phosphate. Barium phosphate is bonded to an ionic bond because the bond is created by giving or taking electrons. 251 rows Calcium Phosphate.
What is the ionic formula for barium and phosphate. Ans- The IUPAC name given to the barium phosphate is barium2. So barium phosphate would be Ba3 PO42.
In this video well write the correct formula for Barium acetate BaCH3COO2To write the formula for Barium acetate well use the Periodic Table a Common. Molar mass of Li3PO4 115794361 gmol Convert grams Lithium Phosphate to moles or moles Lithium Phosphate to grams. Three barium ions each with a charge of 2 would give us six positive charges while two phosphate ions each with a charge of 3 would give us six negative charges.
The phosphate anion is PO4 3-. State Some of the Physical Features of the Salt. Nitrogen is from Group 15 and can form nitride ions N 3.
Ionic Formula Name of Ionic Compound Al 2 O 3 Aluminum Oxide Ba 3 N 2 barium nitride 3 Cs 2 O Cesium Oxide CaO calcium oxide Li 2 O. The barium cation is Ba2. Which of these are ionic compounds.
The formula for Sodium Phosphate Na3PO4. What is the formula for Ba3 PO4 2. Diphosphate the barium phosphate formula is written as Ba 3 PO 4 2.
The phosphate anion is PO4 3-. Know how to determine the chemical formula for ionic covalent. Write the formula for each polyatomic ionic compound shown below.
Barium is an alkaline earth metal and forms B a 2 ions. Sodium Na and one phosphate anion PO 4 3-that are bound through an ionic bond. Calcium nitrite Ca NO22 PubChem.
Therefore the formula is three barium ions to two phosphate ions. The salt that forms will be electrostatically neutral so formula is B a 3 N. It is used in the production of cleaning and bleaching agents as degreaser soaps detergents etc.
Na 2 CO 3 H 3 PO 4 Na 2 HPO 4 CO 2 H 2 O. Name Chemical Formula Ammonium phosphate NH43PO4 Mercury II silicate HgSiO3 Lithium phosphite Li3O3P Manganese II borate Mn3BO32 Barium nitrate. Ba3 PO42 What is the correct formula for the compound formed by Ca2 and NO2.
The barium cation is Ba2. So barium phosphate would be Ba3PO42. Na 2 HPO 4 NaOH Na 3 PO 4 H 2 O.
It has a very high coefficient of thermal expansion and melting point. It is prepared by. It is used to make a sodium and barium polyphosphate combination which is one of the components of hot glasses and other special-purpose glasses.
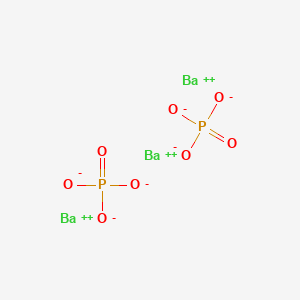
Barium Phosphate Ba3 Po4 2 Pubchem

No comments for "Ionic Compound Formula for Barium Phosphate"
Post a Comment